Research Overview
|
To address the increasing global energy challenges, our group adopts two complementary approaches to harnessing renewable energy (e.g. solar, wind, and hydroelectric): rechargeable batteries and electrocatalysis. We also develop advanced battery technologies for portable energy storage including portable devices and electrical vehicles. Furthermore, we take advantage of electrochemical potential to tackle the challenges of organic synthesis: electrosynthesis.
Our group hosts a number of state of the art experimental and analytic instruments and has access to research facilities at USU campus, USTAR Nanofabrication Center at University of Utah, and national advanced research facility centers. We have advanced skills and techniques to handle the synthesis and characterization of solid materials and molecules (both organic and inorganic) and the development of electrochemical devices (flow batteries, fuel cells, battery devices, electrolyzes, and flow e-reactors). We offer technical services and solution consultation to external users (please contact Professor Liu directly). Please check out our synthesis and testing platforms in the Research Facility page (Link). |
News reports on flow battery research
10/11/2019 ChemistryViews: "Better Electrolyte for Redox-Flow Batteries: pH-Neutral aqueous organic redox-flow battery with an ammonium anthraquinone anolyte" [Link]. Also check out an animated Youtube highlight, www.youtube.com/watch?v=3FIRpdc1-5w 01/04/2019 China Science Daily (中国科学时报) and Sciencenet (中国科学网), “打造可再生能源后备军”. [Link] 11/13/2018 Science Magazine, "Advances in flow batteries promise cheap backup power". Science 2018, 362 (6414), 508-509." [Link] 03/19/2018 Phys.org, "Two better than one: Chemists advance sustainable battery technology". [Link] 01/04/2017 China Science Daily (中国科学时报) and Sciencenet (中国科学网), “科学家研制出高性能水相有机液流电池”. [Link] 02/08/2016 C&EN, "How Redox Flow Batteries Could Stabilize Our Electric Grids". [Link] |
Stationary Energy Storage
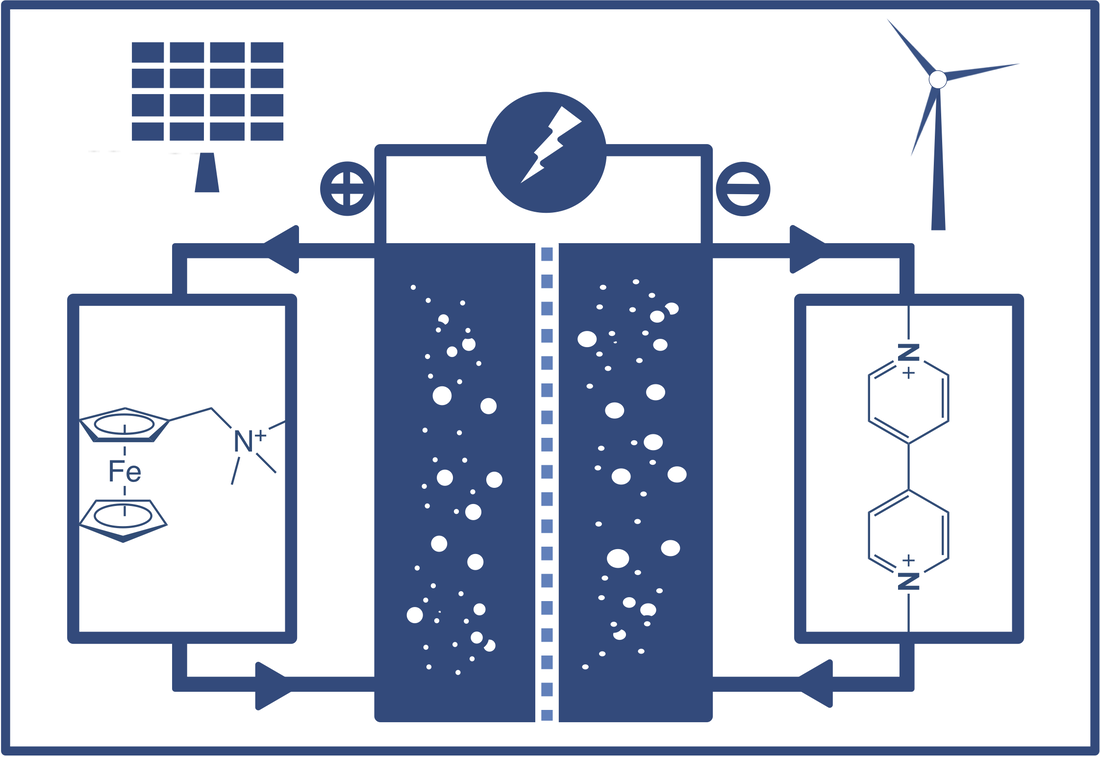
Redox flow batteries are regarded a promising technology for scalable and dispatchable energy storage of renewable energy, balancing electrical grid, and residential and commercial energy storage. However, traditional aqueous inorganic redox flow batteries (AIRFBs) such as vanadium and Zn-bromine AIRFBs have been facing a number of material challenges including expensive raw materials (VOSO4), parasite reactions (hydrogen and oxygen evolution, decomposition of battery components), environmental concerns using corrosive and/or toxic electrolytes (learn more about AIRFBs in our chapter, "Redox Active Inorganic Materials for Redox Flow Batteries", in EBIC) . To address these challenges, we have been developing aqueous organic redox flow batteries (AORFBs) using tunable and sustainable redox organic molecules as electrolyte materials. Specifically, we have developed pH neutral AORFBs using viologen and anthraquinone anolytes and a variety of catholytes including water-soluble ferrocene, TEMPO, ferrocyanide, halides etc. Particularly, the viologen/ferrocynide AORFB represents the most stable and energy dense AORFB to date. To learn more about AORFBs, please reference to our review article (ACS Energy Lett. 2019, 4, 2220-2240).
We have also developed three channel-AORFBs for desalination applications of sea water while reserving energy storage capability. Representative publications: Hu, B.; Liu, T. L.* "Tanking Up Energy Through Atypical Charging", Science. 2021, 6544, 788-789. [Link] Luo, J.; Hu, B.; Hu, M.; Liu, T. L.* "Status and Prospects of Organic Redox Flow Batteries towards Renewable Energy Storage", ACS Energy Lett. 2019, 4, 2220-2240. [Link] Hu, B. (co-1st author); Luo, J. (co-1st author); Hu, M.; Yuan B. Liu, T. L.* "A pH Neutral, Metal Free Aqueous Organic Redox Flow Battery Employing an Ammonium Anthraquinone Anolyte", Angew. Chem. Int. Ed. 2019, 58, 16629-16636. [Link] Luo, J. (co-1st author); Hu, B. (co-1st author); DeBruler C. Zhao, Y., Yuan B. Hu, M. Wu, W. Liu, T. L.* "Unprecedented Capacity and Stability of Ammonium Ferrocyanide Catholyte in pH Neutral Aqueous Redox Flow Batteries", Joule 2019, 4, 1-15. [Link] DeBruler C.; Hu, B.; Moss, J.; Luo, J.; Liu, T. L.* "A Sulfonate Functionalized Viologen Enabling Neutral Cation Exchange Aqueous Organic Redox Flow Batteries towards Renewable Energy Storage", ACS Energy Lett. 2018, 3, 663-668. [Link] DeBruler C. (co-1st author); Hu, B. (co-1st author); Moss, J.; Liu, X. Luo, J.; Sun, Y; Liu, T. L.* "Designer Two Electron Storage Viologen Anolyte Materials for Aqueous Organic Redox Flow Batteries", Chem 2017, 3, 961-978. [Link] Luo, J.; Sam, A.; Hu, B.; DeBruler C. Liu, T. L.* "Unraveling pH Dependent Cycling Stability of Ferricyanide / Ferrocyanide in Redox Flow Batteries", Nano Energy 2017, 42, 215-221. [Link] Hu, B.; DeBruler C., Rhodes, Z.; Liu, T. L.* "Long Cycling Aqueous Organic Redox Flow Battery (AORFB) for Sustainable and Safe Energy Storage", J. Amer. Chem. Soc. 2017, 139, 1207. [Link] |
|
Portable Energy Storage
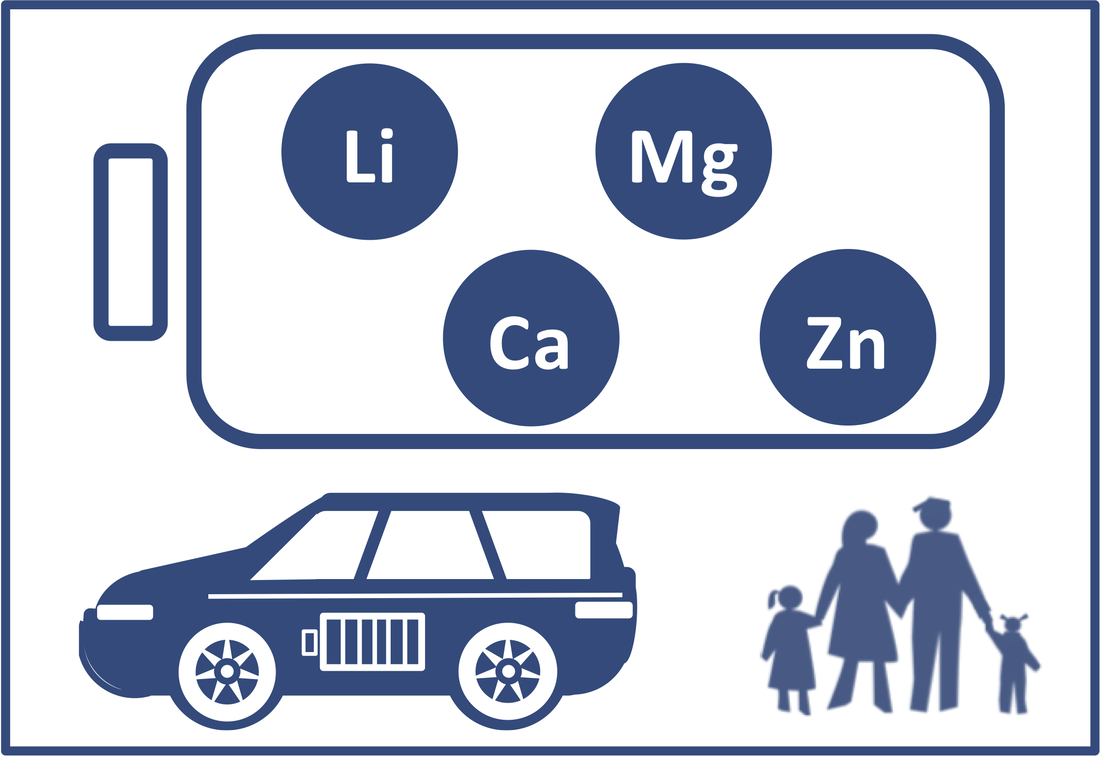
We develop novel battery technologies beyond traditional Li ion and lead acid batteries to seek applications in portable electronic devices and electric vehicles. We primarily emphasize the development of multivalent metal batteries (Mg, Ca, and Zn). We have also invested research efforts in discovering new technologies to advance Li ion and Li metal batteries. We are particularly interested with electrolyte chemistry, electrode materials, and interfacial chemistry between electrolytes and electrode materials. We have made original contributions to the development Mg/Cl complex electrolytes (Gen1, MAC electrolytes and Gen2, MMAC electrolytes, please read our review: Energy Storage Mater 2017, 8, 184-188). Gen2 MMAC electrolytes have been used to develop high energy density Mg/S batteries. We have also advanced the development of Cl-free Magnesium electrolytes using ingeniously designed weakly coordinating anions, which enables the development of high voltage Mg batteries.
Leveraging our expertise in Magnesium electrolyte chemistry, we have also developed calcium electrolytes for high voltage Ca batteries. Representative publications: Bi, Y.; He, S.; Fan, C.; Luo, J.; Yuan, B.; Liu, T. L.* "A Robust Ionic Liquid Magnesium Electrolyte Enabling Mg/S Batteries", J. Mater. Chem. A 2020, 8, 12301-12305. [Link] Sun, J.; Deng, C.; Bi, Y.; Wu, K.; Zhu, S.; Xie, Z.; Li, C.; Amal, R.; Luo, L.; Liu, T. L.*; Wang, D.* "In-Situ Sulfurized Carbon-confined Cobalt for Long-Life Mg/S Batteries" ACS Appl. Energy Mater. 2020, 3, 2516–2525. [Link] Nielson, K. V.; Luo, J. Liu, T. L.* "Optimizing Calcium Electrolytes by Solvent Manipulation for Ca Batteries", Batteries & Supercaps, 2020, 3, 768-772. [Link] Nielson K. V.; Liu, T. L.* "Dawn of Calcium Batteries", Angew. Chem. Int. Ed. 2020, 59, 3368-3370. [Link] Luo, J. (co-1st author); Bi, Y. (co-1st author); Zhang, L.; Zhang, X.; Liu, T. L.* "A Stable, Non-corrosive Perfluorinated Pinacolatoborate Mg Electrolyte for Rechargeable Mg Batteries", Angew. Chem. Int. Ed. 2019, 58, 6967-6971. [Link] Luo, J; .He, S.; Liu, T. L.* "Ternary Mg/MgCl2/AlCl3 Inorganic Mg2+ Electrolytes with Unprecedented Electrochemical Performance for Reversible Mg Deposition", ACS Energy Letters 2017, 2, 1197-1202. [Link] He, S.; Neilson, K. V.; Luo, J..; Liu, T. L.* "Recent Advances on MgCl2 Based Electrolytes for Rechargeable Mg Batteries", Energy Storage Mater 2017, 8, 184-188. [Link]. Liu, T.*; Shao, Y.; Li, G.; Gu, M.; Hu, J.; Xu, S.; Nie, Z.; Chen, X.; Wang, C.; Liu, J. "A facile approach using MgCl2 to formulate high performance Mg2+ electrolytes for rechargeable Mg batteries", J. Mater. Chem. A 2014, 2, 3430-3438. [Link] |
News reports on energy catalysis
05/31/2019 ACS Energy Focus interview, "Role for Standardization in Electrocatalytic Ammonia Synthesis: A Conversation with Leo Liu, Lauren Greenlee, and Douglas MacFarlane". [Link] |
Energy Electrocatalysis
The electrocatalysis program encompasses synthesis and electrochemical studies of bio-inspired (e.g. nitrogenase, CO-dehydrogenase, and hydrogenase) molecular catalysts and hybrid organic/inorganic nano-catalysts for chemical fuel production (H2, CO, alcohols, and ammonia) using base metal catalysts, abundant resources (H2O, CO2, and N2) and renewable energy. We are also interested with electrochemical consumption of chemical fuels such as oxidation of hydrogen and other fuels. At the fundamental level, we want to manage multi-proton-multi-electron transfers to facilitate catalytic energy conversion at optimal rate and energy efficiency. Additionally, we are extremely enthusiastic in fabricating energy device prototypes (electrolyzers and fuel cells) for technical implementation.
Representative publications: Badalyan, A.; Yang, Z.; Hu, B.; Luo, J.; Hu. M.; Liu, T. L. Lance Seefeldt*; "An Efficient Viologen-Based Electron Donor to Nitrogenase", Biochemistry 2019, 58, 4590-4595. [Link] Hu, Bo.; Hu. M.; Seefeldt L.; Liu, T. L.*; "Electrochemical Nitrogen Reduction to Ammonia by Mo2N: Catalysis or Decomposition?", ACS Energy Lett. 2019, 4, 1053-1054. [Link] Liu, T.*; Wang, X.; Hoffmann, C.; DuBois, D. L.; Bullock, R. M. "Heterolytic Cleavage of Hydrogen by an Iron Hydrogenase Model: An Fe-H···H-N Dihydrogen Bond Characterized by Neutron Diffraction", Angew. Chem. Int. Ed. 2014, 53, 5300-5304. [Link] Liu, T.*; DuBois, M. R.; DuBois, D. L.; and Bullock, R. M. "Electrochemical Oxidation of H2 Catalyzed by Ruthenium Hydride Complexes Bearing P2N2 Ligands With Pendant Amines as Proton Relays", Energy Environ. Sci., 2014, 7, 3630-3639. [Link]. |
|
Green Electrosynthesis
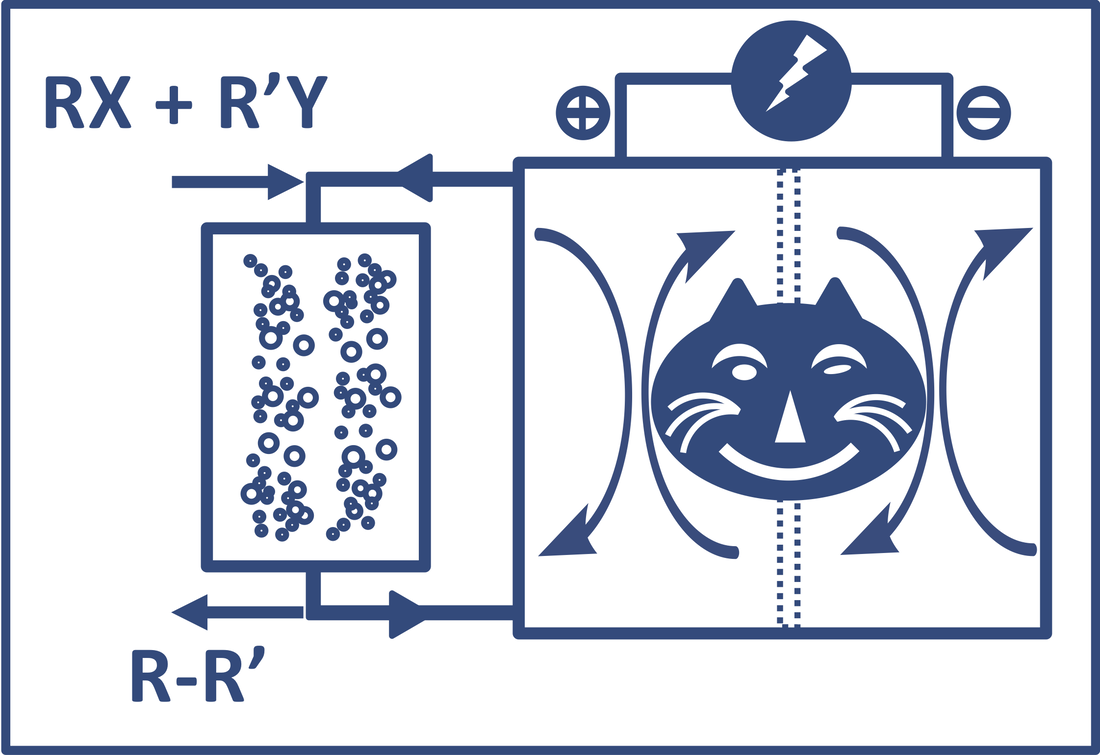
In spite of being known for many decades, until very recently, electrosynthesis has aroused ongoing attention and is believed to have profound impacts on organic synthesis. By precisely controlling redox potential in an electrolyzer cell, substrates or catalysts can be selectively anodically or catholically activated to produce desired reaction sequences. Electrosynthesis not only migrates the use of reactive (even dangerous) oxidants and reductants but it enables access to highly reactive catalytic intermediates which are not easily handled in traditional thermal reactions. Thereby, electrosynthesis represents a green, atomically economical synthetic strategy.
This theme is directed on the development of novel electrosynthesis methodologies to achieve unconventional bond cleavage and formation and enable efficient production of pharmaceutical candidates, agrochemicals, and organic materials. We are particularly interested using inexpensive metals (e.g. Mn, Fe, Ni, Co, W, Mo) to handle catalytic reactions that are traditionally achieved by precious metal (e.g. Rh, Ir, and Pd) based catalysts. To realize “base metals for noble tasks”, we pay particular attention to ligand design and take fully advantage of the ligand second coordination sphere effect and the metal-ligand cooperation effect for highly efficient bond activation and formation. Representative publications: Luo, L.; Hu, B.; Wu, W.; Hu, M. Liu, T. L.* "Ni-Catalyzed Electrochemical C(sp2)−C(sp3) Cross-Coupling Reactions", Angew. Chem. Int. Ed. 2021, 60, 2-12. [Link]; ChemRxiv, 20200206. [Link] Luo, J.; Hu, B.; Sam, A.; Liu, T. L.* "Metal Free Electrocatalytic Aerobic Hydroxylation of Arylboronic Acids", Org. Lett. 2018, 20, 361-364. [Link] |